Revisit TBCK-A Pseudo Kinase or a True Kinase
Introduction
The field of Molecular Biology is constantly uncovering the intricacies of cellular mechanisms and genetic pathways that govern life. One such molecule at the intersection of neurogenetic disorders and cancer biology is TBCK, a gene implicated in regulating protein phosphorylation and cell growth. The study titled “Revisit TBCK-A Pseudo Kinase or a True Kinase” sheds light on TBCK’s dual role as a pseudokinase and its potential involvement in cellular signaling pathways.
This blog explores the critical insights from the study and its implications for Molecular Biology, emphasizing TBCK’s relevance in health and disease.
The Enigma of TBCK: Pseudo Kinase or True Kinase?
TBCK in Cellular Functions
Since its discovery, TBCK has been associated with various biological processes, including:
- Neurodevelopmental Disorders: TBCK mutations are linked to TBCK encephalopathy, a rare autosomal recessive disorder characterized by hypotonia, epilepsy, and intellectual disabilities.
- Cancer Biology: Aberrant TBCK expression impacts pathways like mTOR signaling, suggesting roles in tumor suppression and progression.
Pseudo Kinase Characteristics
The study highlights that TBCK’s kinase domain lacks key motifs required for catalytic activity, such as the HRD and DFG residues. Despite this, TBCK influences protein phosphorylation, indicating indirect or residual kinase-like activity.

Figure 1: DNA sequence alignment between TBCK transcripts. A. Sequence alignment for N terminal sequences of 5 NCBI listed TBCK transcripts using APE DNA editor (https://jorgensen.biology.utah.edu/wayned/ape/); B. Variant 2 exhibits a shorter 5’ UTR sequence compared to variant 1. But they have the same ORF and encode the same protein product.
 Figure 2: DNA sequence alignment between TBCK transcripts. A. Sequence alignment for N terminal sequences of 5 NCBI listed TBCK transcripts using APE DNA editor (https://jorgensen.biology.utah.edu/wayned/ape/); B. Variant 2 exhibits a shorter 5’ UTR sequence compared to variant 1. But they have the same ORF and encode the same protein product.
Figure 2: DNA sequence alignment between TBCK transcripts. A. Sequence alignment for N terminal sequences of 5 NCBI listed TBCK transcripts using APE DNA editor (https://jorgensen.biology.utah.edu/wayned/ape/); B. Variant 2 exhibits a shorter 5’ UTR sequence compared to variant 1. But they have the same ORF and encode the same protein product.
Methodology and Key Findings
Sequence and Structural Analysis
The researchers utilized advanced tools like AlphaFold2 and SMART to analyze TBCK’s structure. Key findings include:
- Splicing Variants: TBCK exhibits alternative splicing, particularly in the kinase domain, leading to functional diversity.
- Motif Deficiency: Compared to canonical kinases like PKA, TBCK lacks critical residues for ATP binding and catalytic activity.
 Figure 3: Protein sequence alignment and putative kinase activity of TBCK. A-C. Sequence alignment for 4 TBCK isoforms using online BLAST tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&BLAST_SPEC=blast2seq&LINK_LOC=blasttab&LAST_PAGE=blastn&BLAST_INIT=blast2seq); D. a dynamic force-directed network for all human kinome and selected categories were displayed via a web application called Coral; E. Heatmap depicting the differential expression of selected genes in the enriched process: Negative regulation of phosphorylation (GO: 0042326).
Figure 3: Protein sequence alignment and putative kinase activity of TBCK. A-C. Sequence alignment for 4 TBCK isoforms using online BLAST tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&BLAST_SPEC=blast2seq&LINK_LOC=blasttab&LAST_PAGE=blastn&BLAST_INIT=blast2seq); D. a dynamic force-directed network for all human kinome and selected categories were displayed via a web application called Coral; E. Heatmap depicting the differential expression of selected genes in the enriched process: Negative regulation of phosphorylation (GO: 0042326).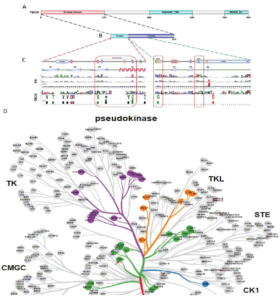 Figure 4: Kinase domain analysis for TBCK. A. A diagram of TBCK including 3 known domains: STYKc; TBC and RHOD; B. N lobe and C-lobe of TBCK kinase domain; C. Detailed motifs and structure for kinase domain of TBCK were visualized via the online database KinOrtho. D. TBCK was listed as a pseudo kinase in a kinase tree.
Figure 4: Kinase domain analysis for TBCK. A. A diagram of TBCK including 3 known domains: STYKc; TBC and RHOD; B. N lobe and C-lobe of TBCK kinase domain; C. Detailed motifs and structure for kinase domain of TBCK were visualized via the online database KinOrtho. D. TBCK was listed as a pseudo kinase in a kinase tree.
Functional Implications
Gene ontology analysis revealed TBCK’s involvement in:
- Protein Phosphorylation: Modulating signaling pathways critical for cell growth and differentiation.
- Cytoskeleton Assembly: Affecting cellular morphology and motility.
These insights underscore TBCK’s multifaceted roles in Molecular Biology.
TBCK in Disease Pathways
Neurogenetic Disorders
Mutations in TBCK are linked to severe neurological conditions:
- TBCK Encephalopathy: Caused by truncating mutations, this disorder disrupts autophagy and cellular metabolism.
- Boricua Mutation: A specific variant associated with profound neurodevelopmental impairment.
Cancer Progression
TBCK’s role in oncology includes:
- Tumor Suppression: Downregulation of TBCK increases sensitivity to chemotherapeutic agents.
- Signaling Modulation: TBCK’s interaction with pathways like STAT3 influences cancer cell proliferation and survival.
Challenges and Future Directions in Molecular Biology
Research Gaps
Despite progress, TBCK’s precise mechanisms remain elusive:
- Kinase Activity: Determining whether TBCK retains basal catalytic functions.
- Protein Interactions: Identifying TBCK’s binding partners to elucidate its role in phosphorylation.
Next Steps
To address these gaps, future research should focus on:
- High-Throughput Screening: Identifying TBCK’s interactome to map its signaling network.
- Functional Assays: Assessing the impact of splicing variants on cellular functions.
- Therapeutic Targeting: Exploring TBCK’s potential as a biomarker for neurogenetic disorders and cancer.
Broader Implications for Molecular Biology
TBCK’s study provides valuable lessons for Molecular Biology:
- Interdisciplinary Insights: Bridging neurobiology and oncology to understand complex diseases.
- Model Development: Using advanced tools like AlphaFold2 to predict protein structures and functions.
- Therapeutic Innovation: Leveraging TBCK’s pathways for drug development and personalized medicine.
Conclusion
TBCK epitomizes the complexity of pseudokinases in Molecular Biology, challenging traditional definitions of enzymatic activity. Its dual roles in neurogenetic disorders and cancer highlight its importance as a research focus. By unraveling TBCK’s functions, scientists can pave the way for novel therapies and deepen our understanding of cellular signaling.
The keywords “Molecular Biology” emphasize the centrality of this field in addressing the unresolved mysteries of TBCK, promising breakthroughs that transcend disciplinary boundaries.
