Enforcement and Enlargement of the Saccharomyces cerevisiae Endoplasmic Reticulum through Artificial Evocation of the Unfolded Protein Response
Introduction
Saccharomyces cerevisiae, commonly known as baker’s yeast, is a model organism extensively used in biotechnology. Researchers have developed a new method to expand the endoplasmic reticulum (ER) in yeast cells by artificially triggering the Unfolded Protein Response (UPR). This method enhances the cell’s ability to produce valuable proteins and lipids, presenting exciting possibilities for biomanufacturing applications. This article explores the process and implications of this breakthrough in yeast engineering.
The Unfolded Protein Response (UPR) and Its Role in ER Function
The ER is the site where cells synthesize and fold secretory proteins and lipids. When the ER experiences stress, cells activate the Unfolded Protein Response (UPR), a protective mechanism that upregulates genes to alleviate stress and restore normal ER function. In S. cerevisiae, the UPR is mediated by the transcription factor Hac1, which is produced when ER stress triggers the splicing of the HAC1 mRNA.
This study demonstrates that by artificially inducing UPR through Hac1 expression in non-stress conditions, yeast cells expand their ER, increasing their capacity to produce high-value compounds like secretory proteins and lipids. For a comprehensive read, check the full text or access the study via DOI link(igmin142).
Methodology: Artificial Induction of UPR in Yeast
To achieve ER enlargement in S. cerevisiae, researchers expressed Hac1 protein using a constitutive promoter. The method led to significant ER expansion, allowing the production of larger lipid droplets and an increase in secretory protein output. However, this artificial UPR also slowed cell growth, suggesting a trade-off between production yield and growth rate.
- Genetic Engineering for Hac1 Expression
Hac1 protein was expressed continuously to stimulate the UPR, leading to enhanced ER structure. Observations under fluorescence microscopy confirmed larger ER and increased lipid storage capacity in these genetically engineered cells. - Balancing UPR for Growth Optimization
Continuous Hac1 expression can impair cell growth, creating a challenge for industrial applications. Researchers explored using mild ER stressors to balance growth and productivity, achieving stable production without excessive growth inhibition. However, additional research is needed to refine this method for large-scale production.
Results and Applications in Biotechnology
Artificial UPR induction led to improved protein secretion and lipid production in S. cerevisiae. These findings open new avenues in biotechnology, where yeast cells could be optimized to produce high-value biopharmaceuticals, like antibodies and functional lipids, more efficiently.
- Lipid Production: The expanded ER allowed for larger lipid droplets, potentially increasing the yield of lipid-based bioproducts.
- Protein Secretion: Enhanced ER capacity also facilitated the secretion of heterologous proteins, making this approach valuable for biopharmaceutical manufacturing.
Future Directions and Challenges
To further optimize this technique, researchers are looking into inducible promoter systems that can control Hac1 expression at specific growth stages, balancing cell mass and production. By identifying genes that contribute to growth inhibition, scientists hope to create yeast strains that grow rapidly while maintaining high UPR levels. Such advancements could transform yeast cells into efficient production platforms for diverse biotechnological applications.
Conclusion
Artificially expanding the ER in S. cerevisiae through the UPR offers a promising approach to enhance yeast productivity in biotechnology. As research progresses, these engineered yeast strains could become invaluable tools for producing biopharmaceuticals and biofuels, potentially redefining sustainable manufacturing.
Tags:
Saccharomyces cerevisiae, Endoplasmic Reticulum, Unfolded Protein Response, Yeast Biotechnology, Protein Secretion, Lipid Production, Molecular Biology, Biomanufacturing.
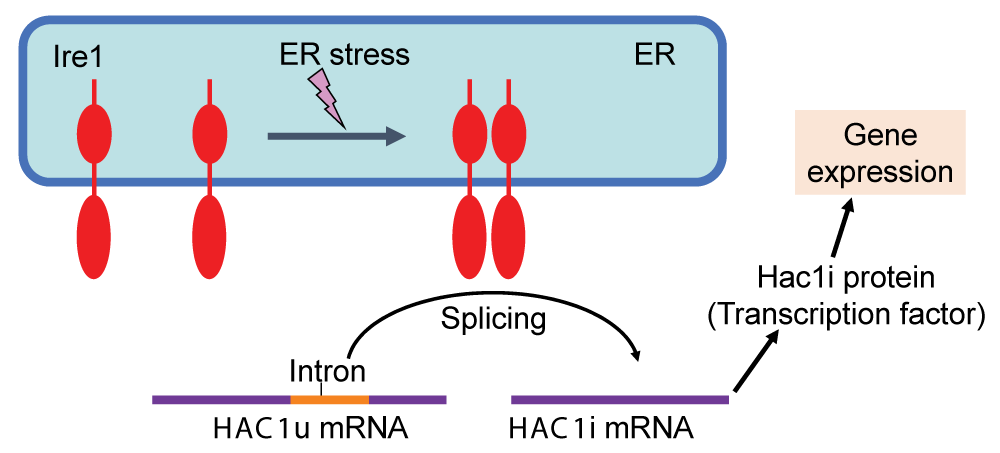
Figure 1: The UPR in S. cerevisiae cells Under non-stress conditions, the HAC1 mRNA (HAC1u) contains the intron sequence and is functionless. Upon ER stress, Ire1 is homo-associated to splice the HAC1u mRNA, yielding the HAC1i mRNA, which is translated to the Hac1i protein to induce gene expression for the UPR.
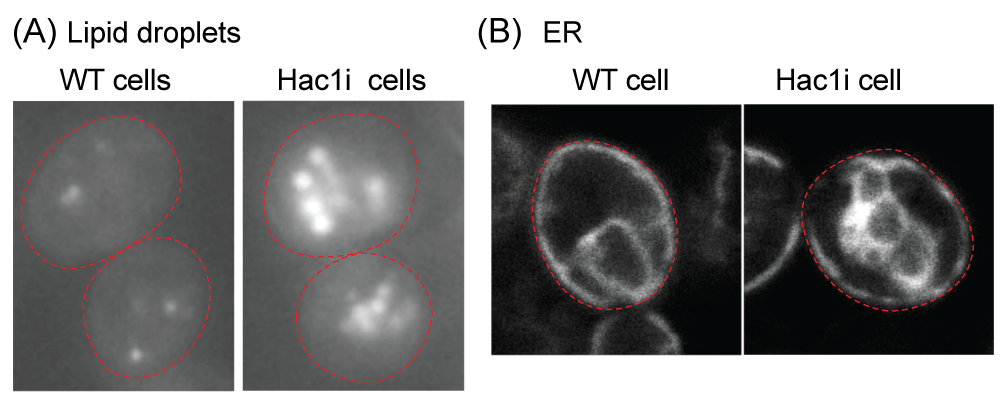
Figure 2: Fluorescence microscopic analysis of S. cerevisiae cells Wild-type S. cerevisiae cells (BY4742 (MATα, ura3, leu2, his3, lys2): WT cell) and their derivative carrying the HAC1i expression plasmid (Hac1i cell) were cultured in yeast standard synthetic complete (SC) medium at 30 °C and fluorescence microscopically observed. To generate the HAC1i expression plasmid, the HAC1i gene was cloned into the Tet-off vector pCM190 [20], leading to inducible expression of the Hac1i protein upon culturing cells in the SC medium. (A) Cells were stained with BODIPY 493/503 to visualize neutral lipids. Hac1i cells carried larger and more abundant lipid droplets than WT cells. (B) The fluorescent ER marker protein, Elo2-mCherry, was expressed from a plasmid that had been created by insertion of the ELO2 gene, which encodes an ER membrane protein Elo2, into the mCherry expression plasmid pYT-TDH3p-PMA1-mCherry [21]. The ER in Hac1i cells was more expanded than in WT cells. Red dashed lines represent the cell outline.
