Biomimetic Synthesis of Calcium Carbonate in Bile in the presence of Amino Acids
Biochemistry – Unraveling the Crystallization of Calcium Carbonate in Bile
Biochemistry offers profound insights into physiological processes and pathologies, bridging our understanding of molecular interactions and clinical manifestations. A compelling example is the study of calcium carbonate crystallization in bile and its implications for gallstone formation. This blog explores research on biomimetic synthesis of calcium carbonate, focusing on the interplay between bile components and amino acids, as revealed in cutting-edge studies.
Introduction
Gallstones, a common global health concern, impact millions and are projected to increase due to metabolic and dietary changes. Gallstone formation is driven by the crystallization of calcium carbonate in bile—a biochemical phenomenon influenced by the presence of bile acids, proteins, and amino acids. By investigating these processes, researchers aim to uncover therapeutic and preventive strategies for cholelithiasis (gallstone disease).
Biochemistry of Calcium Carbonate Crystallization
- Role of Bile Components:
- Pathogenic bile exhibits increased levels of nitrogen, calcium, and magnesium, contributing to the crystallization process.
- Calcium ions serve as nucleation sites, enabling the aggregation of cholesterol and the subsequent formation of gallstones.
- Mechanism of Crystallization:
- Supersaturation of bile with cholesterol initiates nucleation.
- Heterogeneous crystallization, involving calcium salts and bilirubin, catalyzes the process, promoting the formation of calcite and vaterite.
- Impact of Amino Acids:
- Amino acids like glycine and glutamic acid influence the stability and phase composition of calcium carbonate, promoting the crystallization of specific polymorphs such as vaterite.
Experimental Insights
Synthesis of Calcium Carbonate
Experimental studies replicated bile conditions to synthesize calcium carbonate, revealing key findings:
- Polymorph Formation:
- Low bile concentrations favor calcite, while higher concentrations induce vaterite crystallization.
- Organic Inclusions:
- Components like bilirubin and albumin integrate into the calcium carbonate matrix, altering its dissolution kinetics.
Dissolution Dynamics
- Calcium carbonate formed in the presence of bile dissolves slower, attributed to organic inclusions that stabilize the crystal structure.
- The dissolution rate is significantly lower in EDTA compared to saline solutions due to calcium-ion chelation.
Implications for Biochemistry
- Understanding Gallstone Pathogenesis:
- Insights into bile composition and crystallization dynamics illuminate the etiology of gallstones.
- Therapeutic Potential:
- Targeting bile composition through dietary or pharmacological interventions could mitigate gallstone risk.
- Relevance to Broader Biochemistry:
- Findings extend to other biomineralization processes, including bone health and renal stone prevention.
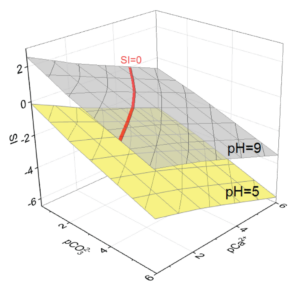 Figure 1: Diagrams of dependence of SI on pCa2+ and pCO32- in a model solution at pH = 5 and 9.
Figure 1: Diagrams of dependence of SI on pCa2+ and pCO32- in a model solution at pH = 5 and 9.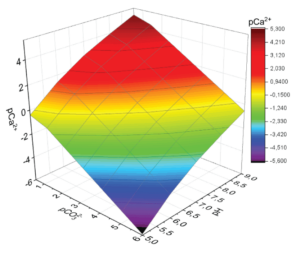
Figure 2: Calcite stability field where Ca2+ complexation is ignored.
- Findings extend to other biomineralization processes, including bone health and renal stone prevention.
Conclusion
The intricate biochemistry of calcium carbonate crystallization in bile provides valuable insights into gallstone formation and potential preventive measures. By bridging experimental and clinical biochemistry, researchers pave the way for innovative solutions to address gallstone disease and related pathologies.
For an in-depth exploration, refer to the detailed HTML article or access the PDF version.
FAQs
- What role does calcium carbonate play in gallstone formation?
Calcium carbonate acts as a nucleation site in bile, facilitating the aggregation of cholesterol and other bile components. This process leads to the crystallization of calcite or vaterite, contributing to gallstone formation. - How do bile components affect calcium carbonate crystallization?
Bile components like bilirubin, amino acids, and albumin influence the stability and structure of calcium carbonate crystals. Their presence alters the crystallization process, favoring certain polymorphs such as vaterite under specific conditions. - What impact do amino acids have on the crystallization process?
Amino acids like glycine and glutamic acid stabilize vaterite formation and affect the phase composition of calcium carbonate. This interaction modifies the dissolution kinetics and stability of the crystals in bile. - How does calcium carbonate formed in bile dissolve differently?
Calcium carbonate formed in bile dissolves more slowly due to organic inclusions, which stabilize the crystals. This reduced dissolution rate is particularly evident in EDTA solutions, where calcium-ion chelation occurs. - What are the broader implications of this research in biochemistry?
Beyond gallstone formation, this research offers insights into biomineralization processes relevant to bone health, renal stone prevention, and material science. It also highlights potential therapeutic strategies for managing bile composition to prevent gallstones.
