Efficient Room Temperature Ethanol Vapor Sensing by Unique Fractal Features of Tin Oxide
Revolutionizing Ethanol Vapor Sensing with Tin Oxide Fractals
Advances in gas-sensing technology are crucial for applications in industries like food processing, breweries, and chemical production. Recent research by Rupali Nagar and Vishal Kamathe from the Nanomaterials for Energy Applications Lab highlights the development of an innovative ethanol vapor sensor that operates at room temperature using fractal structures of tin oxide (SnO2)(igmin150)【Fulltext†source】【DOI†source】.
The Unique Properties of Fractals in Sensing
Fractals, self-similar structures found in nature, are known for their complex geometries that provide extensive surface areas and connectivity. These properties are invaluable for enhancing the performance of gas sensors. The researchers successfully synthesized tin oxide fractals using a sol-gel method coupled with microwave irradiation—a cost-effective and straightforward approach(igmin150).
The resulting SnO2 fractals exhibited dendritic structures that maximize surface interactions and adsorption sites, essential for gas detection. This unique morphology enables efficient sensing at ambient conditions, making it suitable for real-world applications where power conservation is paramount(igmin150).
Fabrication and Testing Methodology
The team prepared SnO2 fractals by mixing tin chloride pentahydrate and urea, followed by microwave exposure at 595 watts. The precipitate was then dried at 70 °C, creating tin oxide structures with sizes ranging from 45 ± 5 nm (as-synthesized) to 12 ± 2 nm (after annealing). X-ray diffraction (XRD) confirmed the crystalline structure, while FTIR spectroscopy verified functional groups, such as O-Sn-O and Sn-OH(igmin150).
The sensor was coated onto a glass substrate and connected using silver epoxy. Testing involved exposing the sensor to ethanol vapor concentrations ranging from 20 to 100 ppm. The average response and recovery times were impressive, clocking in at 18 ± 3 seconds and 22 ± 5 seconds, respectively, even at room temperature(igmin150).
Performance Highlights
The SnO2 fractal sensor demonstrated outstanding ethanol vapor detection capabilities due to:
- High Surface Area: The dendritic morphology provided abundant sites for gas adsorption and interaction.
- Rapid Response and Recovery: The sensor’s performance featured an average response time of about 18 seconds, facilitating real-time monitoring.
- Room-Temperature Operation: Unlike many metal oxide sensors that require heating, this sensor operates efficiently at ambient temperature, reducing energy consumption(igmin150)【Fulltext†source】.
Future Prospects
The implications of this research extend beyond ethanol sensing. The innovative use of fractals in gas sensors could be adapted for other volatile organic compounds and environmental monitoring systems. Future developments could involve scaling the technology for industrial deployment and exploring additional fractal materials for diversified sensing applications(igmin150).
Conclusion
Nagar and Kamathe’s study represents a leap forward in sensor technology, showcasing how nature-inspired fractal structures can be harnessed for efficient and eco-friendly gas detection. This work is a testament to the potential of innovative material design in addressing modern technological challenges.
Tags: Ethanol Sensing, Tin Oxide, Fractal Structures, Room-Temperature Sensors, Gas Detection Technology.
Figure 1: Recreated from ‘Growth of US sensor market. Source: Grand view research, [1], available at https://www.grandviewresearch.com/industry-analysis/gas-sensors-market
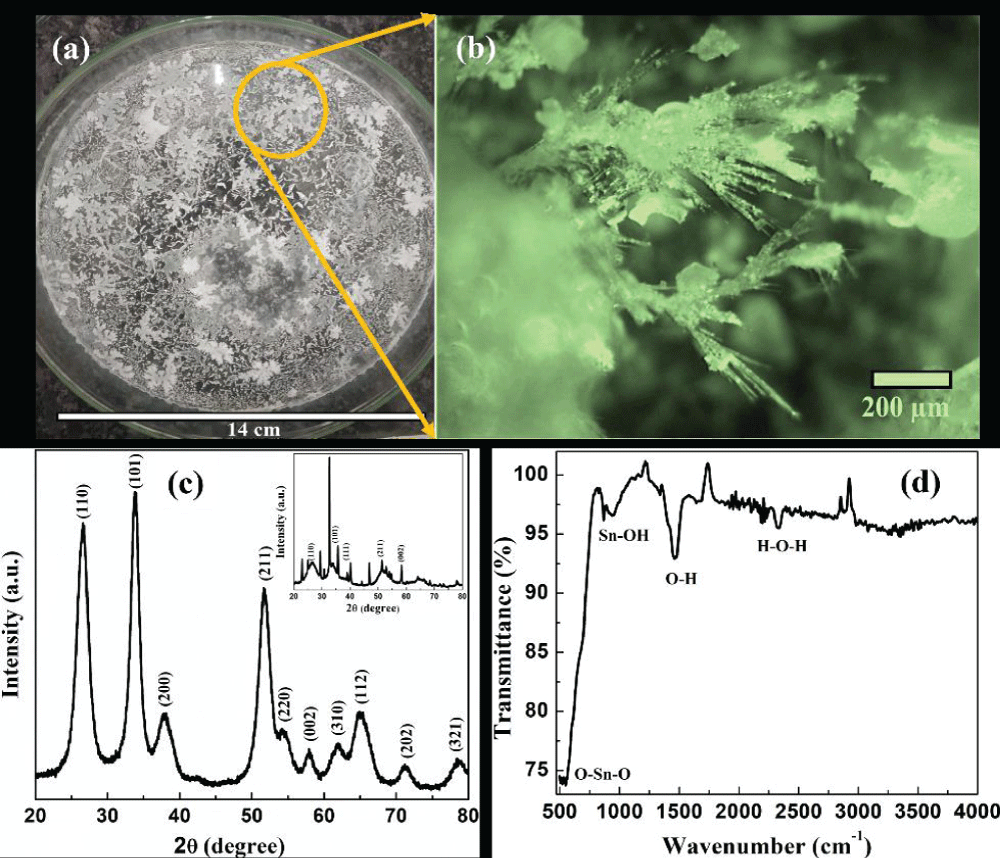
Figure 2: Tin oxide fractals grown on (a) large scale glass substrate, (b) digital optical image showing the dendritic growth, (c) x-ray diffractogram of annealed sample. Inset shows the x-ray diffractogram of as-synthesized fractal sample and (d) FTIR spectrum of tin oxide fractals.
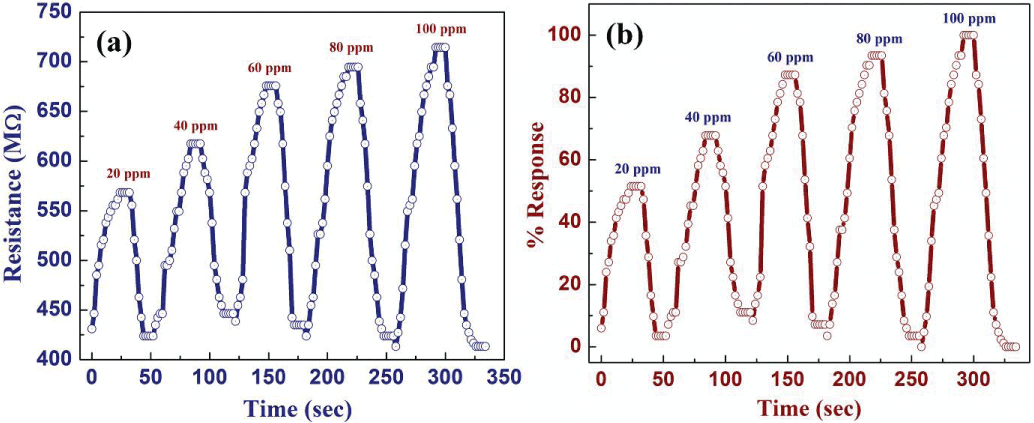
Figure 3: Sensing response of tin oxide sensor under ambient conditions (a) change in resistance of the sensor with exposed ethanol vapor concentration from 20-100 ppm (b) normalised percentage response for 20-100 ppm ethanol vapor concentration.
