Modeling of Cr3+ doped Cassiterite (SnO2) Single Crystals
Unlocking the Mysteries of Cassiterite (SnO2) Single Crystals: The Role of Cr³⁺ Doping
In the pursuit of advanced materials for scientific and industrial applications, the doping of crystals plays a critical role in enhancing their properties. One recent breakthrough in this domain is the modeling of Cr³⁺-doped Cassiterite (SnO₂) single crystals, a topic extensively explored in the research paper by Maroj Bharati, Vikram Singh, and Ram Kripal.
This blog dives into the details of the study, offering insights into the use of Cr³⁺ ions in improving the optical, structural, and magnetic properties of SnO₂, and how this aligns with the broader fields of Biostatistics and Molecular Biology.
Introduction to Cassiterite and Doping
Cassiterite (SnO₂) is a semiconducting oxide known for its optical transparency, mechanical hardness, and stability under heat. These attributes make it a promising material for applications in:
- Gas Sensors
- Solar Cells
- Supercapacitors
- Transparent Conductors
However, doping Cassiterite with Cr³⁺ ions introduces a new dimension of functionality. By substituting Sn⁴⁺ ions with Cr³⁺ ions, researchers aim to refine its magnetic and electronic properties.
The Science Behind Cr³⁺ Doping
Why Cr³⁺?
Chromium ions (Cr³⁺) are well-suited for doping due to their ionic radius (0.0615 nm), which closely matches that of Sn⁴⁺ ions (0.069 nm). This enables them to occupy lattice positions in the crystal structure with minimal distortion.
Superposition Model (SPM)
The study employs the Superposition Model (SPM) to calculate Zero-Field Splitting (ZFS) and Crystal Field (CF) parameters. These calculations provide insights into the optical energy bands and the lattice distortions caused by Cr³⁺ ions.
Key Findings of the Study
- Optical Energy Band Calculations Using the Crystal Field Analysis (CFA) Program, the study calculated optical transitions for Cr³⁺ ions in SnO₂. The results align closely with experimental data, validating the theoretical models.
- Zero-Field Splitting (ZFS) Parameters
- The study achieved an accurate determination of ZFS parameters for Cr³⁺ ions, considering distortion in the SnO₂ lattice.
- ZFS values significantly improve understanding of the material’s magnetic properties.
- Structural Insights Cr³⁺ ions replace Sn⁴⁺ ions at octahedral sites in the crystal lattice, resulting in localized distortions that enhance material properties.
- Applications in Magnetic Devices The findings have implications for designing Single Molecule Magnets (SMMs) and Single Ion Magnets (SIMs), paving the way for innovations in quantum computing and data storage.
Broader Implications in Biostatistics and Molecular Biology
While the study primarily focuses on material science, its implications extend to Biostatistics and Molecular Biology through:
- Data Modeling Techniques: The precision modeling methods used for SnO₂ crystals can inspire similar approaches in molecular simulations.
- Structural Biology: Understanding ion substitutions in crystal lattices parallels how molecular substitutions affect biological structures.
Experimental Methodology
The study employed advanced techniques to ensure the accuracy of its findings:
- Electron Paramagnetic Resonance (EPR): Used to investigate local symmetry and spin properties of Cr³⁺ ions.
- Infrared Microscopy: Provided detailed views of lattice distortions.
- Crystal Field Analysis (CFA) Program: Calculated CF and ZFS parameters, ensuring theoretical consistency.
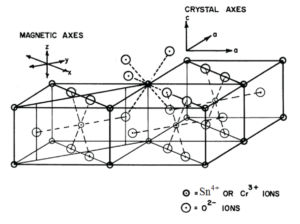 Figure 1: The crystal structure of SnO2 together with the symmetry-adopted axis system (SAAS).
Figure 1: The crystal structure of SnO2 together with the symmetry-adopted axis system (SAAS).
Comparative Analysis
Traditional vs. Modern Approaches
The study highlights the superiority of modern modeling techniques over traditional methods like the Point Charge Model (PCM). The SPM offers a more nuanced understanding of ZFS and CF parameters, making it indispensable for such research.
Cr³⁺ in Other Hosts
The behavior of Cr³⁺ ions in SnO₂ is compared to their behavior in other hosts like LiNbO₃ and TiO₂, revealing universal trends and unique properties.
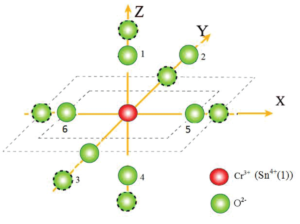 Figure 2: Visual depiction of the local environment (dotted circles show positions after distortion).
Figure 2: Visual depiction of the local environment (dotted circles show positions after distortion).
Future Directions
The study opens up numerous avenues for exploration:
- Expanding Doping Elements: Investigating other ions to complement Cr³⁺ and further enhance SnO₂ properties.
- Industrial Applications: Applying findings to develop efficient sensors, energy devices, and magnetic storage systems.
- Integration with Biostatistics and Molecular Biology: Leveraging the modeling approaches for biological simulations and molecular design.
Conclusion
The modeling of Cr³⁺-doped SnO₂ single crystals represents a significant milestone in materials science. By combining advanced computational methods with experimental validation, this study not only enhances our understanding of crystal behavior but also establishes a framework for interdisciplinary applications, including Biostatistics and Molecular Biology.
As research progresses, the integration of these findings into practical devices will bridge the gap between theoretical science and real-world innovation.
References
FAQs
What is the focus of the study on Cr³⁺ doped Cassiterite (SnO₂) single crystals?
The study investigates the impact of Cr³⁺ doping on the crystal field (CF) and zero-field splitting (ZFS) parameters of Cassiterite (SnO₂) using the Superposition Model (SPM).Why is Cr³⁺ chosen as the dopant for SnO₂ single crystals?
Cr³⁺ ions have a similar ionic radius to Sn⁴⁺ ions, allowing them to substitute Sn⁴⁺ with minimal structural distortion, thereby altering the material’s optical and magnetic properties.What applications can Cr³⁺ doped SnO₂ crystals have?
These crystals have potential uses in solid-state lighting, gas sensors, supercapacitors, dye-sensitized solar cells, and single-molecule magnet systems.What computational methods were used in the study?
The study employed the Crystal Field Analysis (CFA) program and the Superposition Model (SPM) to calculate ZFS and CF parameters, alongside theoretical modeling for energy states.What were the key findings of the study?
The calculations revealed that Cr³⁺ ions replace Sn⁴⁺ ions in the SnO₂ lattice with orthorhombic symmetry, and the theoretical ZFS and CF parameters aligned well with experimental data, supporting the material’s suitability for advanced industrial and scientific applications.
